Abstract
Background: Umbralisib, a selective PI3Kδ and casein kinase-1epsilon (CK1ε) inhibitor, is pharmacologically distinct from other PI3K inhibitors and is administered orally once daily. Ublituximab is a novel anti-CD20 monoclonal antibody glycoengineered for enhanced antibody dependent cellular cytotoxicity (ADCC) that targets a unique epitope on CD20. The UNITY-NHL trial (NCT02793583) is a multicenter, open-label, registration-directed Phase 2 study designed to evaluate the safety and efficacy of the umbralisib + ublituximab (umbra+ubli, U2) combination in patients with previously treated NHL . Umbra has been recently approved for the treatment (tx) of previously treated marginal zone lymphoma (MZL) based on reported data (Fowler JCO 2021), and the U2 combination has been shown to be active with a manageable safety profile in patients with relapsed/refractory (R/R) NHL (Lunning Blood 2019). In contrast with other PI3Kis, there has been a low incidence of immune-mediated toxicities with umbra, possibly attributable to enhanced selectivity for the PI3Kδ isoform as well as inhibition of CK1ε. Herein, we present results from the MZL cohort treated with U2.
Methods: Eligible patients had histologically confirmed MZL (splenic, nodal, extranodal) and were R/R to ≥1 prior lines of tx which must have included an anti-CD20. Patients were treated with U2: umbra was given orally at 800 mg once daily until progression or unacceptable tolerability and ubli was administered intravenously on Days 1, 8, and 15 of Cycle 1, on Day 1 of Cycles 2-6, and on Day 1 every 3 cycles until Cycle 24 in 28-day tx cycles. The primary endpoint of the study was overall response rate (ORR) as assessed by an independent review committee according to the revised IWG criteria (Cheson 2007). Secondary endpoints included duration of response (DoR), progression-free survival (PFS), time to response (TTR), and safety.
Results: 72 MZL patients were enrolled and received U2. The median age was 70 years (range 40-89), 53% were female, 74% had stage III or IV disease, and 25% were refractory to their immediate prior therapy. Patients had received a median of 2 prior regimens (range 1-9). As of the cutoff date, 41 patients remain on tx.
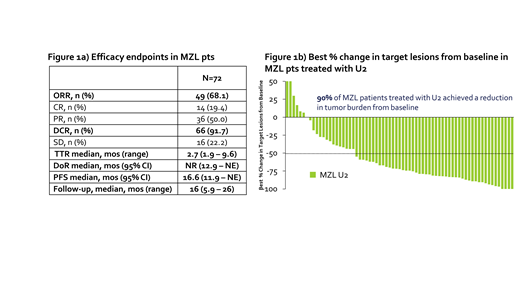
The ORR was 68%, with 19% achieving complete response (CR). At a median follow-up of 16 months, median DoR was not reached (12 - not estimable). 90% of patients had a reduction in disease burden from baseline while on U2. Figure 1a depicts efficacy data for U2, and Figure 1b shows the best % change in target lesions from baseline for MZL patients treated with at least 1 dose of U2.
The most common adverse events (AEs) were diarrhea (all grades: 49%, grade ≥3: 13%), nausea (all grades: 30%, grade ≥3: 0%), and fatigue (all grades: 36%, grade ≥3: 6%). Other AEs of interest included non-infectious colitis (all grades: 2.8 %, grade ≥3: 2.8%) and rash (all grades: 11%, grade ≥3: 0%). Four patients (5.6%) discontinued due to an AE.
Conclusions: U2 was highly active in patients with R/R MZL, with improved efficacy when compared to a prior cohort of MZL patients treated in this study with umbra mono (Fowler JCO 2021). The safety profile of the U2 combination was manageable, with a relatively low incidence of immune-mediated toxicities and AE-related discontinuations.
Chavez: Bristol Myers Squibb: Speakers Bureau; AstraZeneca: Research Funding; BeiGene: Speakers Bureau; Epizyme: Speakers Bureau; MorphoSys: Speakers Bureau; Karyopharm Therapeutics: Consultancy; Merck: Research Funding; Adaptive: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy; Abbvie: Consultancy; Kite/Gilead: Consultancy. Goldschmidt: AbbVie: Consultancy, Research Funding. Samaniego: Imbrium: Membership on an entity's Board of Directors or advisory committees; Arog: Research Funding. Wrobel: Janssen: Honoraria, Speakers Bureau; Roche: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; BeiGene: Honoraria, Speakers Bureau. Cavallo: ROCHE: Membership on an entity's Board of Directors or advisory committees; Gilead: Speakers Bureau; Servier: Speakers Bureau. Fonseca: Sanofi: Honoraria; Karyopharm: Honoraria; Epizyme: Honoraria; Dava Oncology: Honoraria; Celgene/BMS: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Abbvie: Honoraria. Drgona: Abbvie: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau; Sandoz: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Teva: Honoraria; Viatris: Honoraria. Hawkes: Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel and accommodation expenses, Research Funding, Speakers Bureau; Merck KgA: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Specialised Therapeutics: Consultancy; Bristol Myers Squib/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Speakers Bureau; Regeneron: Speakers Bureau; Antigene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Merck Sharpe Dohme: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees. Levi: AbbVie: Consultancy, Research Funding. Miskin: TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Sportelli: TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. O'Connor: Myeloid Therapeutics: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Mundipharma: Consultancy; Nomocan: Consultancy; Kymera: Consultancy, Current equity holder in publicly-traded company; TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company; Dren: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Fowler: Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, TG Therapeutics and Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; BostonGene, Corp: Current Employment, Current holder of stock options in a privately-held company. Zinzani: ROCHE: Other, Speakers Bureau; NOVARTIS: Consultancy, Other, Speakers Bureau; GILEAD: Other: Advisory board, Speakers Bureau; KYOWA KIRIN: Other, Speakers Bureau; SANDOZ: Other: Advisory board; BMS: Other: Advisory board, Speakers Bureau; Incyte: Other, Speakers Bureau; JANSSEN-CILAG: Other: Advisory board, Speakers Bureau; CELLTRION: Other: Advisory board, Speakers Bureau; VERASTEM: Consultancy, Other: Advisory board, Speakers Bureau; SERVIER: Other: Advisory board, Speakers Bureau; TAKEDA: Other: Advisory board, Speakers Bureau; Beigene: Other, Speakers Bureau; MSD: Consultancy, Other: Advisory board, Speakers Bureau; TG Therapeutics: Other: Advisory board, Speakers Bureau; EUSAPHARMA: Consultancy, Other, Speakers Bureau; ADC Therap.: Other.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal